Abstract
Introduction:Therapy-related MDS (t-MDS), defined as MDS occurring after previous exposure to chemotherapy or radiation therapy, constitutes 10-20% of all MDS diagnoses. The latency period between exposure and development of t-MDS is imprecise, making it challenging to report. T-MDS patients tend to have higher-risk disease than de novo MDS patients and worse outcomes. Despite this, t-MDS patients are often excluded from therapeutic clinical trials. We analyzed all therapeutic MDS clinical trials from 1999-2017 to assess the representation of t-MDS patients.
Methods: We performed a search of all therapeutic clinical trials for adults (>18 years) with MDS using clinicaltrials.gov. This included all completed, actively recruiting, ongoing but not recruiting, terminated, or suspended trials (Table 1). Clinical trials were classified into three groups based on eligibility criteria: A: trials excluding subjects with t-MDS; B: trials not indicating exclusion or inclusion of t-MDS subjects; or C: trials specifically including t-MDS subjects. We reviewed any published results (manuscripts and meeting abstracts) for all completed trials in each group and collected the de novo MDS vs the t-MDS patient accruals for all completed clinical trials and the estimated accrual of these patients in actively enrolling clinical trials. The type of sponsor (cooperative groups, academic institutions versus pharmaceutical sponsors) was also compared across all groups for completed and recruiting trials using the N-1 Chi-Squared test.
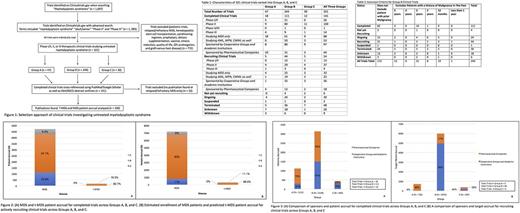
Results: Our initial search yielded 1093 trials; 760 trials were excluded from further analyses as they were not therapeutic trials for untreated MDS patients. Clinical trials studying conditioning regimens and outcomes of stem cell transplantation, prophylactic treatments, relapsed/refractory MDS, dietary supplementation, and only quality of life measurements were excluded (Fig 1). This left 321 trials classified into Groups A (14%, n=47), B (76%, n=244) and C (9%, n=30). The majority of trials were completed (44%, n=141), recruiting (17%, n=54), and terminated (15%, n=48) (Table 1). The eligibility criteria of Group B trials were noteworthy in that 71 trials excluded patients with a history of prior malignancy within the past one (8%, n=19), two (3%, n=8), three (13%, n=31), and five (2%, n=13) years; thus, possibly excluding some t-MDS patients (Table 2).
Out of 141 completed clinical trials, 109 publications were available for analysis (21 abstracts, 88 manuscripts); 14% of publications belonged to Group A, 78% to Group B, and 8% to Group C. Based on these publications, a total of 4,719 MDS patients were enrolled across completed therapeutic trials in all three groups. Only 3.8% (n = 182) of these patients had t-MDS (Fig 2A). Out of 22 studies that enrolled t-MDS patients, only four discussed t-MDS outcomes in their discussion or conclusions; one concluding t-MDS to have similar outcomes to their de novo counterparts (Prebet, Thomas, et al. 2016). Fifty-three ongoing clinical trials aim to accrue 7, 197 patients over the next 5-10 years (Fig 2B). Based on the completed clinical trials data, it is predicted that only 309 t-MDS patients (4.2%) will be accrued (Fig 2B). Out of 1115 patients accrued in Group A, 73% (N = 814) were enrolled in completed trials sponsored by pharmaceutical companies whereas 23% (N = 301) were sponsored by corporate groups and academic institutions (p < 0.0001, Figure 3A). Further, for a target accrual of 397 patients in recruiting clinical trials, pharmaceutical sponsors were far more likely to conduct clinical trials specifically excluding t-MDS patients than cooperative groups or academic institutions (56% vs 44%, p = 0.0007) (Fig 3B).
Conclusions: A majority of completed therapeutic clinical trials for MDS patients exclude or fail to specifically recruit t-MDS patients, who represent 10-20% of newly diagnosed MDS patients but less than 5% of subjects enrolled in completed or ongoing clinical trials. Pharmaceutical sponsors were twice as likely to conduct clinical trials excluding t-MDS patients than cooperative groups and academic institutions. As t-MDS patients represent a significant portion of newly-diagnosed MDS patients, future MDS trials should be more inclusive to more closely reflect the MDS population at-large. Cooperative group trials are critical in this effort to recruit a representative MDS patient population.
Borate: Novartis, Jazz: Consultancy, Speakers Bureau. Sekeres: Celgene: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal